Many production and manufacturing facilities still manually test for microbiological contamination. Counting Colony Forming Units (CFUs) is the standard method to detect contaminants and monitor reference control strains. To do this, technicians must process a large number of samples and record results manually, leading to data capture errors that ultimately compromise data integrity.
LabTwin’s voice-activated digital lab assistant helps improve quality control and prepare for quality audits by allowing technicians to easily record large and complex data sets, in real time, at the testing site, simply by talking. Our digital lab assistant lowers the risk of missing critical and threatening microbial growth in manufactured products.
We recently made quality control and quality assurance even easier with LabTwin’s Voice-Activated Table. This new feature eliminates the time-consuming and labor-intensive process of manually entering results into tables and forms. With LabTwin’s digital lab assistant, technicians can now track, in real time, how their data is recorded in table or written format, thus lowering error rates and improving quality control.
LabTwin allows real-time automated data collection, so technicians and managers can immediately review results and make real-time data-driven decisions.
Use LabTwin’s Voice-Activated Table feature to:
- Count and document CFUs
- Record experimental conditions such as temperature, media batches, reagents, and water supplies
- Monitor equipment parameters such as temperature, humidity and oxygen levels
- Analyze the efficiency of current protocols and methods
- Identify biofilms on equipment, surfaces and instruments
- Perform Preservation Efficacy Tests (PET)
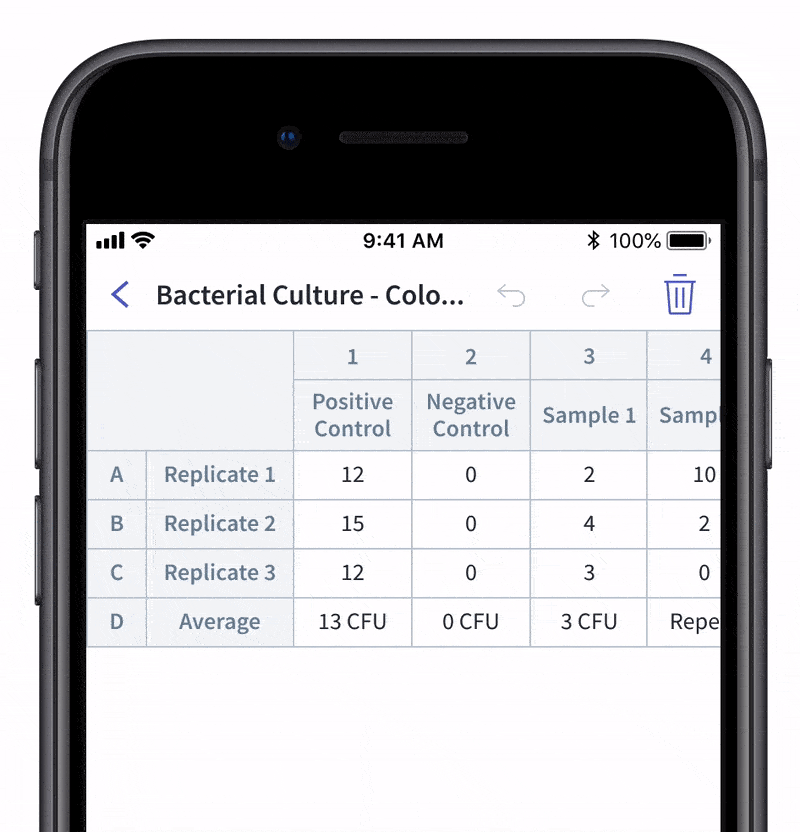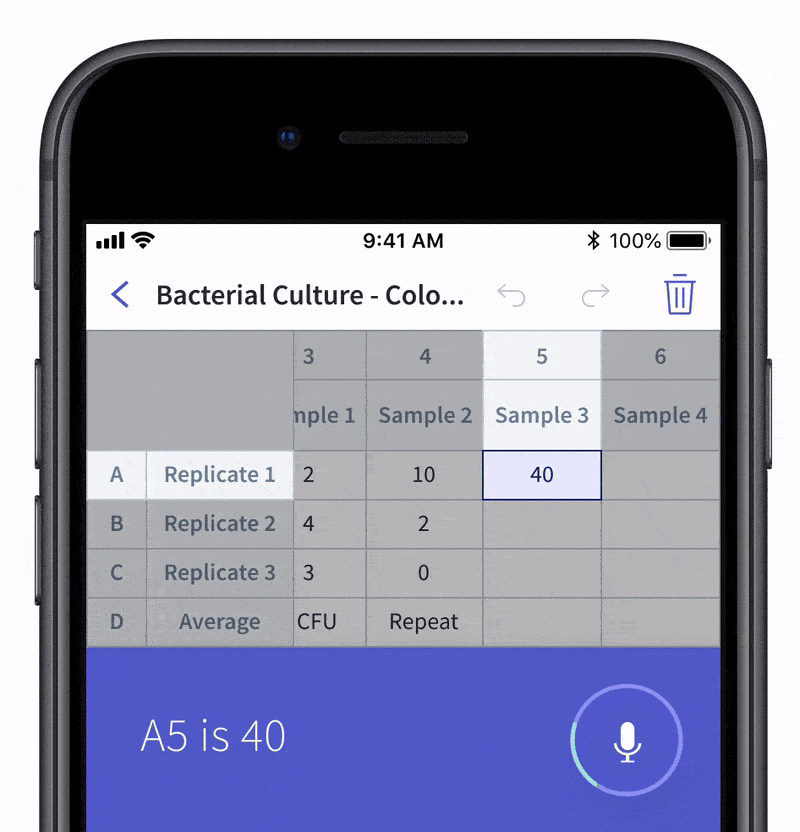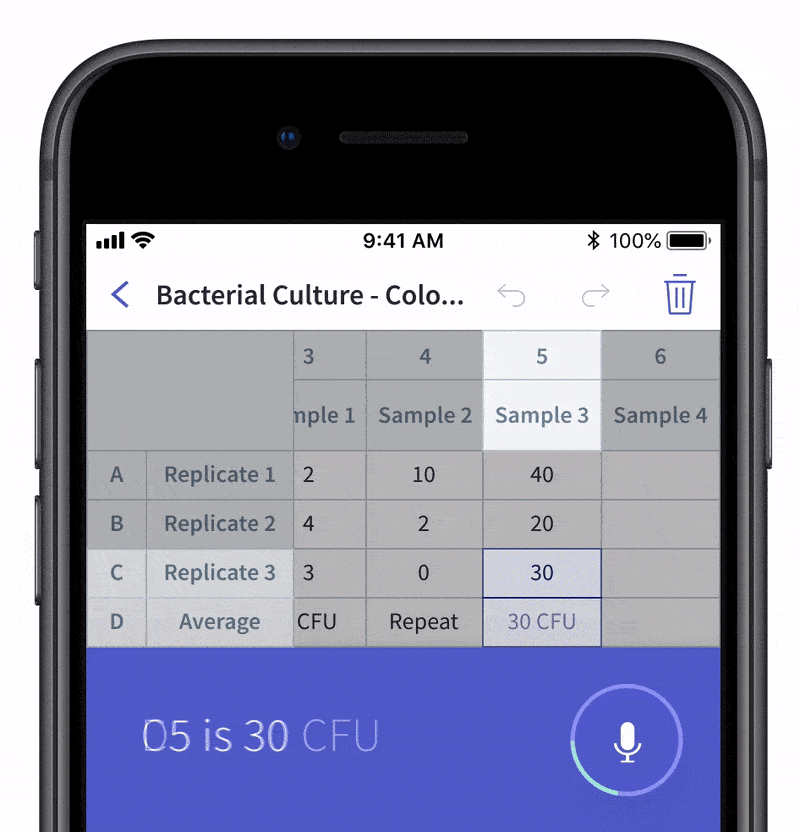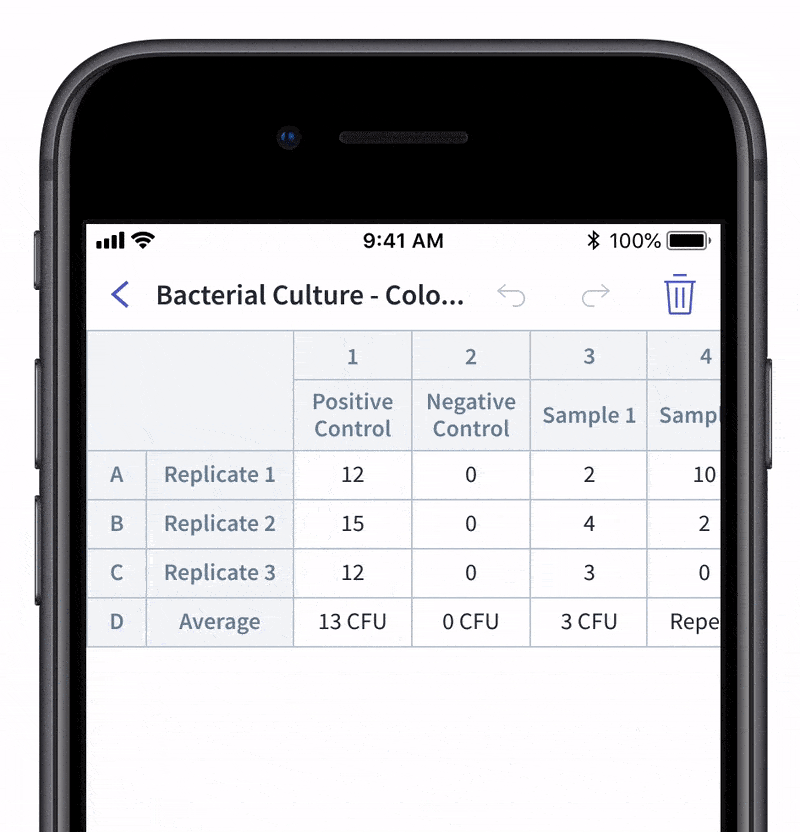
With LabTwin's Voice-Activated Table you can focus on sampling, counting and analyzing your results while fulfilling cGMP regulations and preventing expensive investigations of Microbial Data Deviations (MDD) and Out Of Specification (OOS) errors.











