In preclinical drug development, in vivo studies are a mandatory step in the process of turning lead compounds into drug candidates. Regulatory agencies require in vivo safety and efficacy data from animal models before approving novel candidates for human clinical trials. While animal studies are an essential part of ensuring that new drugs and medicines are safe for human use, they come with significant animal welfare concerns and research costs. According to European Commission reports, an estimated 192 million animals are used every year in drug development studies. Moreover, preclinical in vivo studies cost $12.7 billion in 2018 and this figure is expected to reach $24 billion by 2027.
The importance of documenting in vivo studies
One of the keys to improving animal welfare and reducing in vivo research costs is proper documentation. By capturing truly contemporaneous, accurate and complete data and metadata from every experiment, pharmaceutical labs can identify errors or protocol deviations, improve experimental reproducibility and lower the number of animals required to complete a study.
Animal welfare in research
Understandably, there has been increasing concern for the welfare of animals used in biomedical experiments. There are strict regulatory guidelines covering the experimental use of animals. Wherever possible, research organizations are encouraged to follow the Three Rs Principle, and replace animal experiments with other models, reduce the numbers of animals used in experiments and refine the experimental techniques to minimize harm. Animal Ethics Committees must approve and oversee in vivo animal experiments to ensure regulatory compliance.
Documentation of animal experiments is a legal requirement. Thorough, accurate documentation is also a vital part of proving regulatory compliance and improving animal welfare. SOPs must be validated and all in vivo experimental procedures must be precisely documented and easily accessible for audits. It must also be possible to track and trace every event in each animal’s life from birth to death. Veterinarians may at any time inspect the documentation or collect samples to attest to the animal welfare. Violations of the regulations can lead to a loss of the animal experimentation license for the lab.
By collecting all possible experimental data and metadata, organizations can also enrich datasets and potentially find ways to reduce the number of animals required to produce robust preclinical safety and efficacy data.
“Preclinical research, especially work that uses animal models, seems to be the area that is currently most susceptible to reproducibility issues.”
Francis Collins
NIH Director
Drug development failure rate and the reproducibility crisis
In 2011, Bayer Healthcare researchers reported that only 21% of drug target validation studies could be fully replicated. A 2012 study published by Amgen researchers found that 90% of in vivo oncology studies were irreproducible.
The economic impact of irreproducible animal studies on preclinical research has been estimated at $28 billion annually. As a 2014 Sigma-Aldrich survey identified, “Poor Documentation” is responsible for 34% of experimental irreproducibility.
Improving documentation would reduce the barriers to reproducing experimental methods and therefore save billions of dollars each year.
“In our labs, 85% of experiments are not reproducible. Our last attempt to reproduce experiments sourced form academia caused massive financial damage as we spent 8 hours a day for 6 months on it.”
LabTwin Enthusiast
Vice President In Vivo Pharmacology
Challenges and solutions
In vivo experiments last weeks or months and during this time, various documentation must be completed and transferred between the different team members involved in the study. Due to animal welfare regulations, tested animals need to be monitored every day, resulting in significant documentation often captured in lab notebooks or simple sheets of paper before being digitized. Furthermore, multiple experiments are often performed in parallel and it is essential to keep track of each study-related document.
Scientists estimate that 15% of their total working time is spent on documentation and between 1 and 4 hours are spent every day transcribing notes into Excel or an electronic lab notebook (ELN).
By streamlining documentation with LabTwin’s voice-activated digital lab assistant, scientists are able to handle animals without interruptions, thus reducing the animals’ stress. LabTwin’s digital assistant also reduces cognitive load or the need for an extra notetaker by allowing scientists to voice observations, and experimental conditions, during surgeries. Furthermore, the digital assistant saves time in report creation as all notes are automatically digitized, sorted based on experiment and stored in one central place.
General workflow
A classic in vivo study contains several steps from study design to the final study report. LabTwin supports most of these steps, by empowering data capture and access directly at the point of experimentation and by facilitating collaboration during the study.
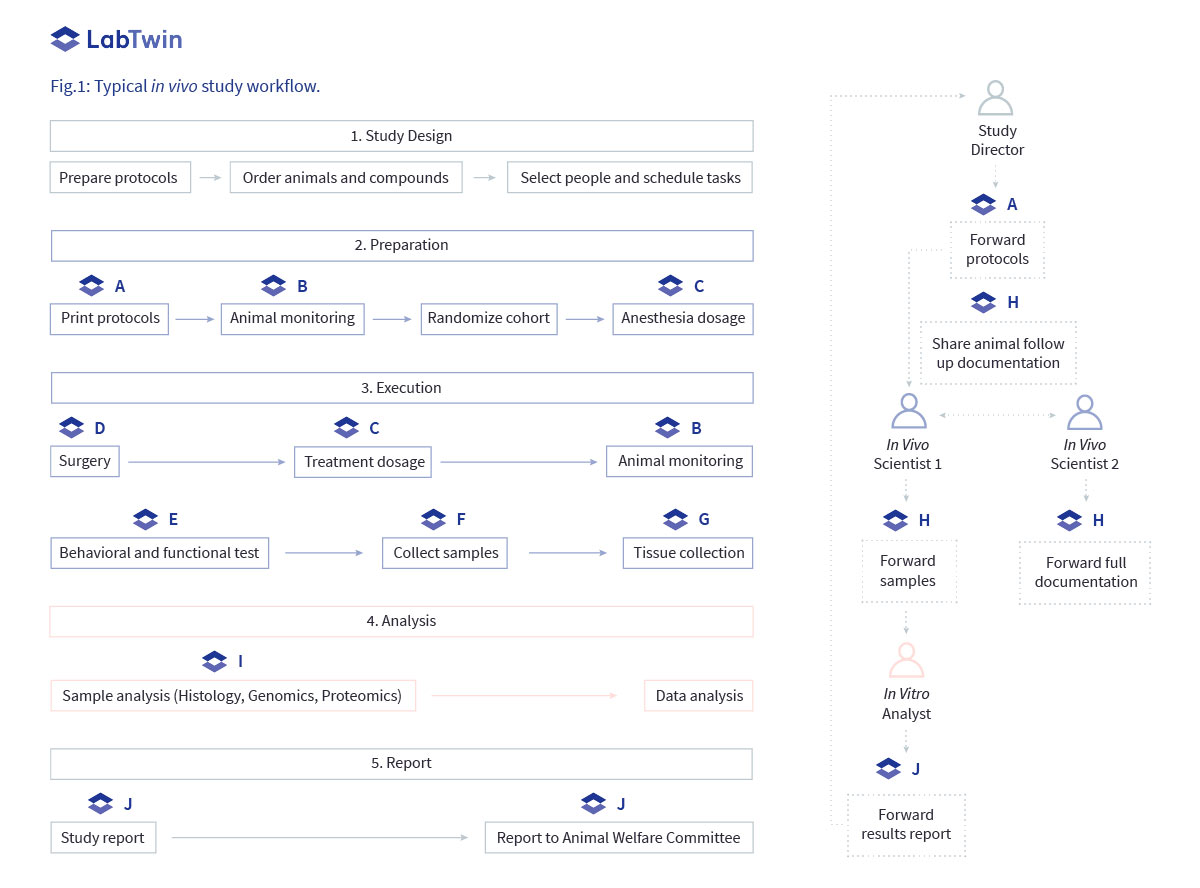
Sharing protocols (A)
Each in vivo study is generally governed by a study director in charge of the design, coordination and final report.
Once the study is designed, protocols are passed on to the laboratory scientists who will perform the experiments. Several different SOPs may be used for each study, such as the surgical procedure, preparation and administration of any drugs or compounds, and sample collection protocols. Scientists usually print these protocols and bring paper copies into the lab.
Instead, each of these protocols can be directly uploaded in the LabTwin Web Platform and be instantly accessible to bench scientists via the Mobile App. LabTwin can read out SOPs to scientists, giving step-by- step verbal instructions during the experiment. Any voice notes or results from lab instruments are automatically linked to each step, simplifying report generation at the end of the experiment.
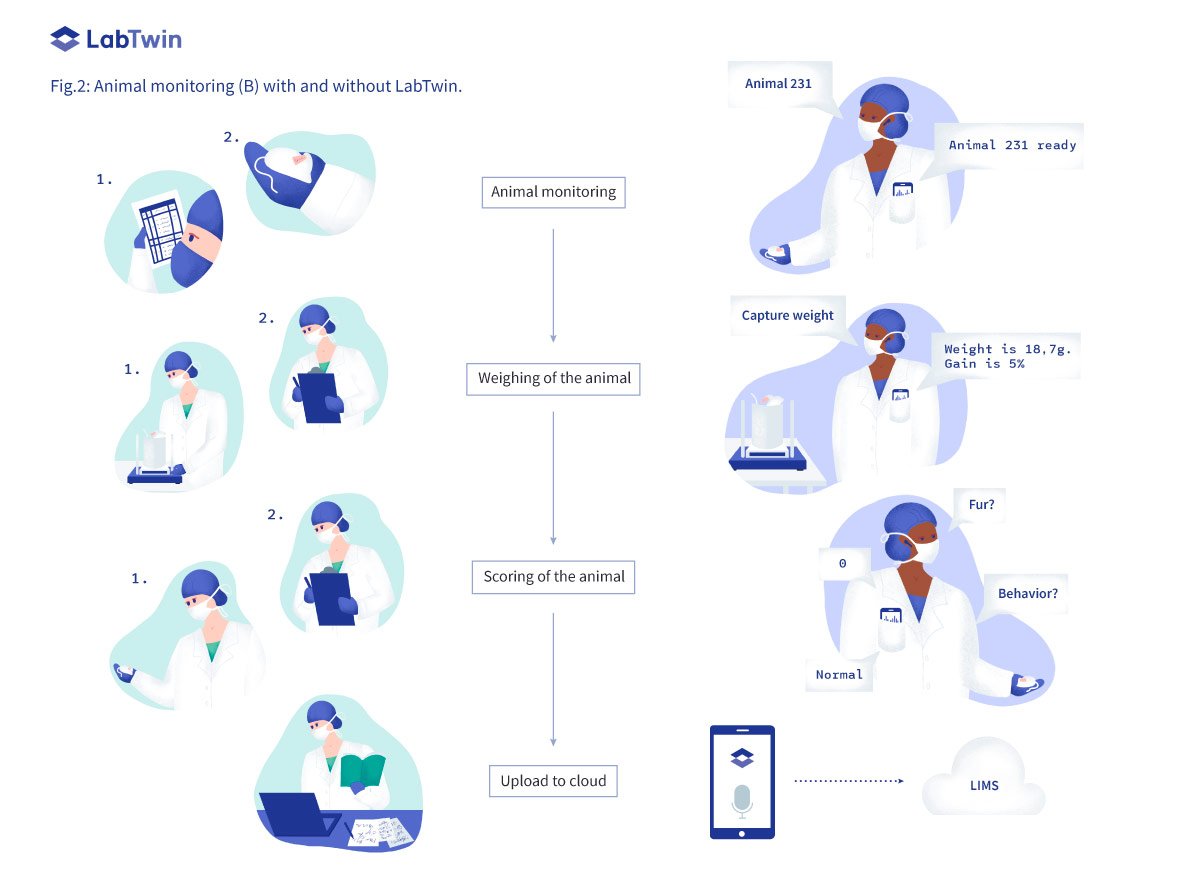
Animal monitoring (B)
According to regulatory guidelines, regular monitoring of animal welfare is mandatory for every in vivo study. Monitoring is often a daily task which is very time-consuming. Multiple parameters, such as the animal’s weight, external appearance or social isolation, must be measured or scored. An acceptable range is specified for each value. Any score outside of these ranges, for example a reduction of more than 20% of the original weight, represents an endpoint for the experiment. When an endpoint is reached, the animal must be sacrificed, and the reason documented.
Usually, each animal has its own score sheet that scientists carry with them into the lab in order to report the welfare parameters. The score sheets are then either stored in a physical folder, scanned and uploaded in the cloud, or manually entered into a laboratory information management system (LIMS). These data must be accessible at any time as organizations can be subject to unannounced inspections from veterinary inspectors or an animal ethics committee.
By integrating with lab devices such as a balance, LabTwin allows direct capture of the original value from the device. The digital assistant can record the weights of each animal and automatically calculate gain or loss of weight. LabTwin can also warn scientists if endpoint values are reached. Scientists can complete the rest of the welfare score sheet by voice, and LabTwin will automatically digitize these notes and upload them into the LIMS. This ensures the database is constantly up-to-date and data is always accessible if an- other scientist needs to take over the monitoring or an inspector wishes to see the data. By streamlining this daily task and avoiding duplicate documentation, LabTwin saves scientists’ time while reducing the risk of data loss and empowering collaboration.
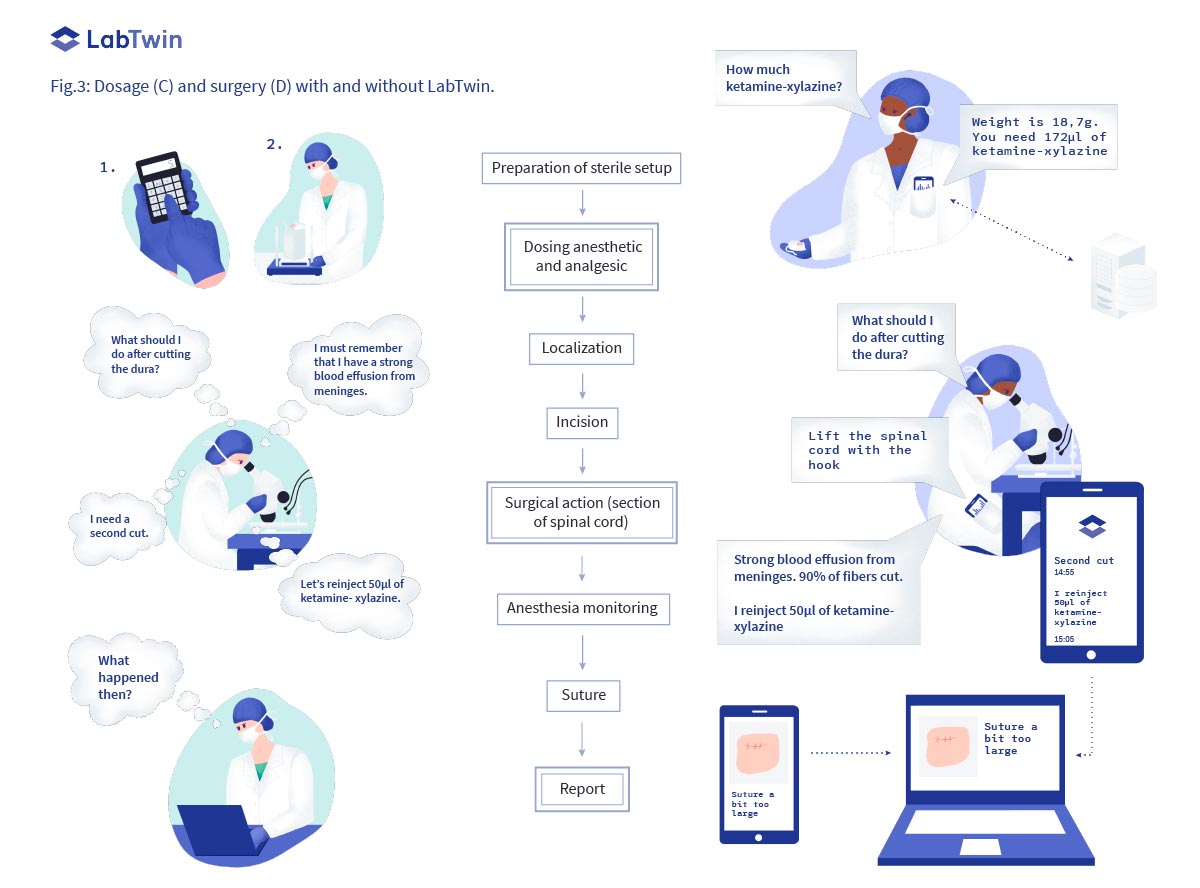
Anesthesia/treatment dosage (C)
When it is time to anesthetize an animal before surgery or test a potential drug candidate, the correct dose must be precisely calculated based on the animal’s weight. This requires scientists to weigh the animal, re- cord the weight and calculate the appropriate dose either mentally or with a calculator.
As LabTwin’s digital lab assistant integrates with balances and supports calculations, it is the perfect tool to streamline drug dosing. With LabTwin, animal weight is directly captured from the balance and entered in a digital table where the correct drug volume is automatically calculated and read out to the scientist.
Surgery (D)
Surgeries such a spinal cord transection, stem cell transplantation or medical device implantation, require intense concentration and are performed in sterile conditions. It is not possible to manually record data while performing such delicate procedures. Therefore, scientists must remember any important observations until the end of the surgery when they have a chance to take notes. This creates cognitive overload, leads to miss- ing or forgotten data and reduces the quality of the dataset. To avoid this, a second person is often monopolized to take notes.
During delicate in vivo procedures, LabTwin operates as the second notetaking person, capturing observations, experimental conditions and protocol deviations in real time. LabTwin automatically adds time stamps to each note, precisely recording the timeline of events. LabTwin users report an increase in the accuracy and quality of documentation, and a reduction of cognitive fatigue when using the app.
In parallel to voice or written notes, pictures are an important documentation tool for surgeries, and help to accurately capture details such as the position of an implant. LabTwin allows direct integration of annotated pictures into experimental notes, reducing the spread of documentation across different platforms.
“My surgeries last 1 hour and I had to keep every note in my head for that whole time.”
LabTwin user
In Vivo Scientist, Top 10 Pharma Company
Behavioral and functional tests (E)
Assessing the impact of a treatment on the animal behavior or some specific physiological function is usually done by running performance tasks and scoring the animal. For example, after spinal section, locomotion can be evaluated by placing the animal in an open-field, examining its gait and using a simple six-point scale to score it in which 0 is no movement and 5 is normal walking.
Scoring a full cohort can be easily achieved by dictating to LabTwin the score value and additional information, automatically filling up a table while running the test. This allows direct digitization of the results, prevent data loss, and keep the focus and animal handling.
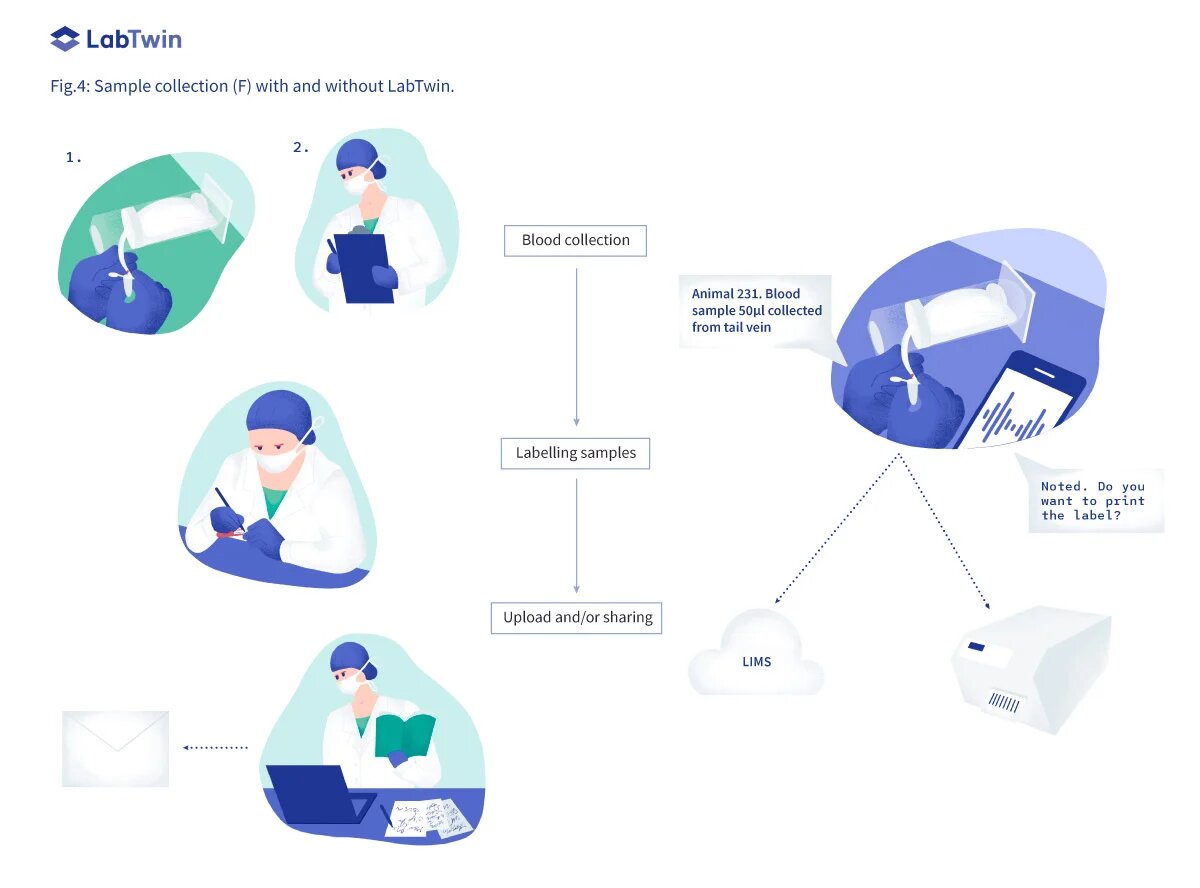
Sample collection (F)
After administration of a new treatment, scientists may collect biological samples, such as blood, urine, cerebrospinal fluid or tissues from biopsies, in order to monitor how the treatment impacts biomarkers. For each sample, the date and time of collection, ID of the animal, name of the scientist and route of collection need to be reported and linked to the sample ID. In many labs, scientists manually record these details on paper then later enter the data into their LIMS or lab database. Such double documentation wastes time and increases the risk of human error.
By integrating with the LIMS, LabTwin can capture all necessary details during sample collection, automatically create a new entry for the sample and print the corresponding label. Sample collections becomes seamless and LabTwin users report that fewer interruptions during animal handling decreases the stress of the animals. Furthermore, real-time digitized documentation at the point of experimentation avoids double documentation and reduces the risk of human error.
“With LabTwin, I can focus more on my work and generate less stress on the animal by handling it without interruption.”
LabTwin user
In Vivo Researcher, Top 10 Pharma Company
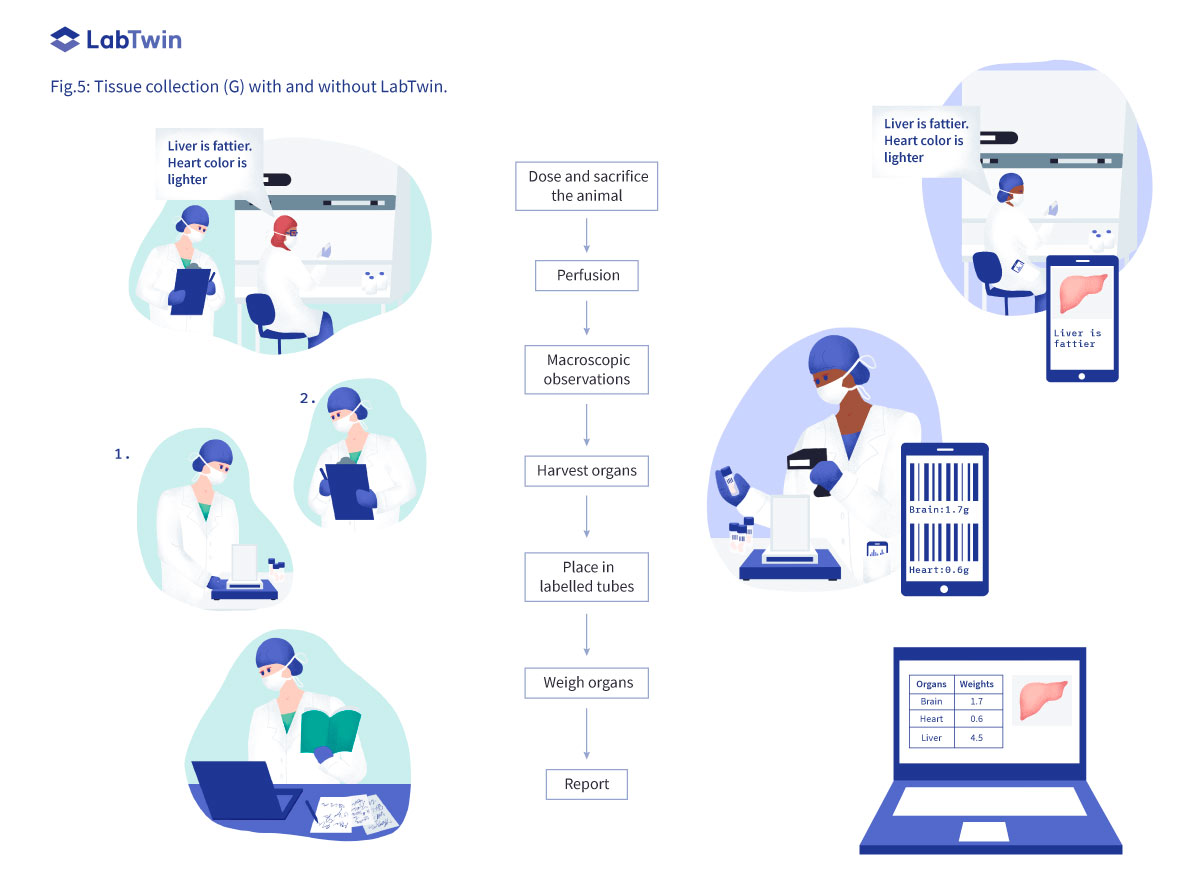
Tissue Collection (G)
At the end of the experiment, each animal is sacrificed and organs are harvested for toxicology assays. Each organ has to be weighed, measured and assessed for any macroscopic changes that could relate to potential drug toxicity. Collected tissues are then placed in labeled tubes containing paraformaldehyde or directly frozen in liquid nitrogen. Scientists must wear appropriate personal protective equipment (PPE), and may use a fumehood, to protect from paraformaldehyde toxic vapors. Manual documentation is very challenging in this environment. Here again, documentation is either postponed or accomplished by a dedicated notetaker.
With LabTwin, scientists can voice real-time observations during tissue collection and directly integrate annotated pictures into their notes. Scientists can identify each organ by voice and LabTwin will automatically create a table listing each organ and its corresponding weight captured from the connected balance. LabTwin labels all data according to experiment so it is easy to gather data into a report to share with colleagues and collaborators. LabTwin users reported saving at least 1 hour while harvesting tissue by eliminating manual documentation.
Shared documentation (H)
Animal studies can take months and usually involve collaboration between several scientists with different expertise. Monitoring animal welfare status is a daily task often shared between team members. When samples need to be handed over to the analysis team, all associated details must also be accessible. Unfortunately, documentation is often scattered on different platforms, between paper forms and excel sheets, and some- times exchanged by emails. Lack of digitization prevents fast data retrieval and increases the risk of data loss.
By operating as a central hub between data generation at the bench and data storage on the cloud, LabTwin facilitates data sharing and reduces the risk of data loss and data silos.
“So far, if I want to look for an information from a study, I would search the number of the animal and read through the scanned notes.”
LabTwin user
Senior Research Scientist, Top 10 Pharma Company
Sample analysis (I)
Once samples and tissues have been collected from the animal, several assays can be performed to detect and quantify biomarkers. These assays are usually run by a different team and the documentation needs to be both accessible and editable.
With LabTwin’s digital lab assistant, all sample information is digitized and uploaded into the cloud or LIMS. Analysts can then retrieve any necessary information by querying LabTwin directly at the bench. Analysts can also follow protocols with verbal instructions and capture results by voice with LabTwin. So far, LabTwin users estimate saving 1.5 hours per in vivo bioanalysis workflow.
Final study report (J)
At the end of a study spanning several months, the study director has the difficult task of gathering all documentation, assay results and analysis into a comprehensive report. This report must include any deviations from the protocols approved by the Animal Welfare Committee, as well as the cause of death for every animal used in the study.
Report generation usually takes hours.
LabTwin helps scientists save hours during report creation by having documentation already centralized and in digital format. Moreover, during any unannounced inspections or audits, data about each animal can be easily accessed, helping to make the process more transparent.
“All but a handful of Nobel Prize winners for physiology and medicine have been animal researchers,”
Chris Magee
Head of Policy and Media, Understanding Animal Research
Conclusion
Although there has been an effort to reduce the use of animals in pharmaceutical research, in vivo animal studies are still required for both drug discovery and preclinical safety and efficiency testing.
To improve animal welfare and increase experimental reproducibility, a real collective effort needs to be made to enrich documentation.
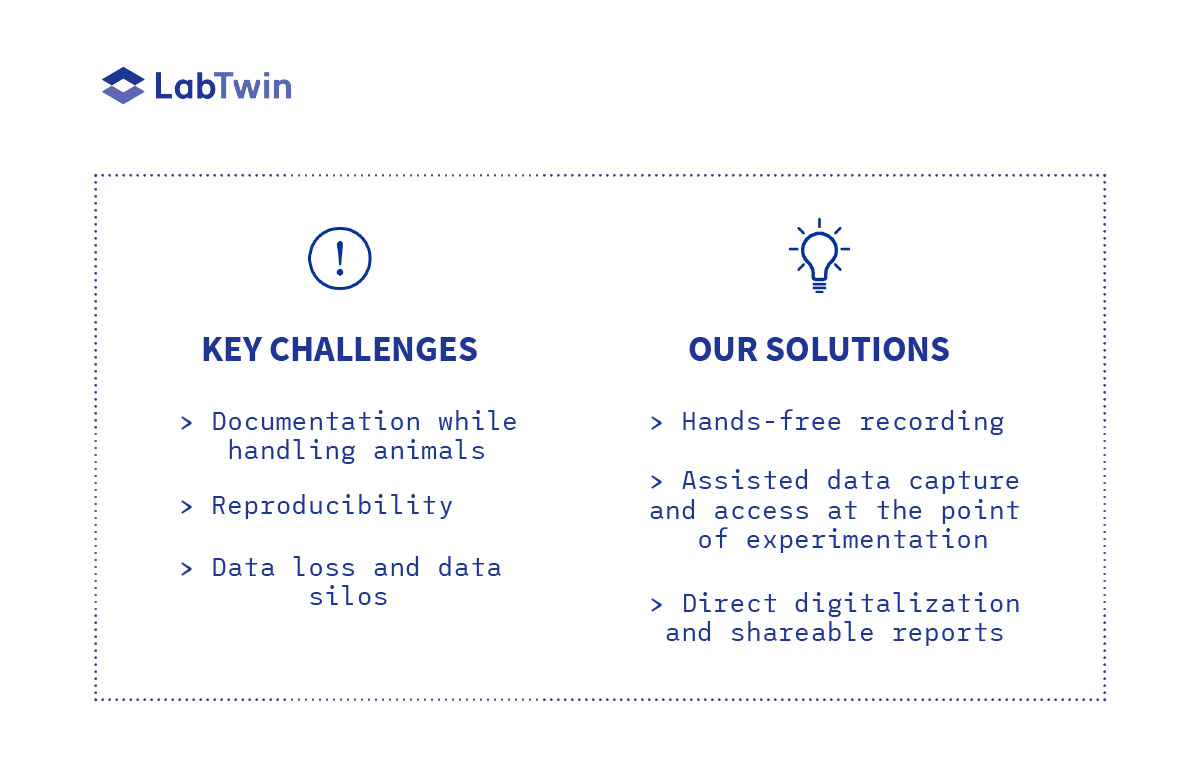
Challenges in animal research documentation come from three main reasons:
1. The inability to document while handling animals
2. The length of animal studies increasing the risk of data loss
3. The multitude of collaborators involved in one study who need to share documentation
LabTwin addresses each of these challenges by bringing hands-free documentation with immediate digitization into the lab. With LabTwin, scientist are able to collect all their different data in one single place and share it on the cloud in a comprehensive report, saving time in the lab and bypassing the need for an extra person to take notes.
Richer notes captured at the point of experiment are a strong asset that make experiments more reproducible. Users estimate that LabTwin increases their experimental reproducibility by 50%. This, in turn, reduces the need for repeated experiments due to lost data and therefore, reduces animal use and improves animal welfare.



