We all expect that the medicine we receive is safe and effective. For pharmaceutical products to meet these requirements, regulatory authorities, such as the European Medicines Agency (EMA) or the U.S. Food and Drug Administration (FDA), ensure that drug manufacturing processes follow current Good Manufacturing Practice (cGMP).
Microbiological testing plays an important role in the pharmaceutical manufacturing process, from the development of a biopharmaceutical product right through to post-approval marketing. The growing microbiological testing market is expected to reach US$5 billion in the next three years.
Indeed, before each batch of a vaccine or drug is released, it must first be tested to ensure it conforms to safety and efficacy standards. This usually includes multiple tests, each following precise cGMP regulations. For each test assay, a validated Standard Operating Protocol (SOP) must be used and a quality control (QC) analyst must document completion of every step of the protocol. All documentation must be initialed and include precise times and dates. Several steps require validation by a second person and the final document has to be reviewed and approved by QC and quality assurance (QA) managers. Documentation is a mandatory and onerous part of pharmaceutical QC processes.
By allowing real-time, hands-free documentation, LabTwin’s voice-activated digital lab assistant provides an exclusive solution for documenting under cGMP regulations. QC analysts estimate they save, on average, 30% of their time by using LabTwin. Furthermore, analysts report that the digital lab assistant improves traceability and data compliance. LabTwin enables automatic and complete digitization of GMP forms, which is a considerable asset in the FAIRification process.
cGMP - MICROBIOLOGICAL ASSAYS
A variety of assays exist to ensure biopharmaceutical product quality and integrity at each stage of product development. Here we describe some commonly used assays and highlight the hurdles analysts face while running them.
SAFETY TESTING OF STERILE SAMPLES: THE ENDOTOXIN TEST (GEL CLOT METHOD)
Some products, such as injectable drugs or vaccines, must be sterile because microbial contamination could affect the therapeutic effect of the product or cause harmful infections. Microbial contamination can originate from human intervention but also from raw materials, including water and plant-based materials.
The bacterial endotoxin test is a common sterility test. It detects the presence of Gram-negative bacteria. These bacteria express lipopolysaccharides (also called endotoxins) in their outer cell membrane. Endotoxins induce many adverse pathological effects such as fever, or endothelial cell damage, and can lead to septic shock. Endotoxins can remain active even after bacterial death due to sterilization.
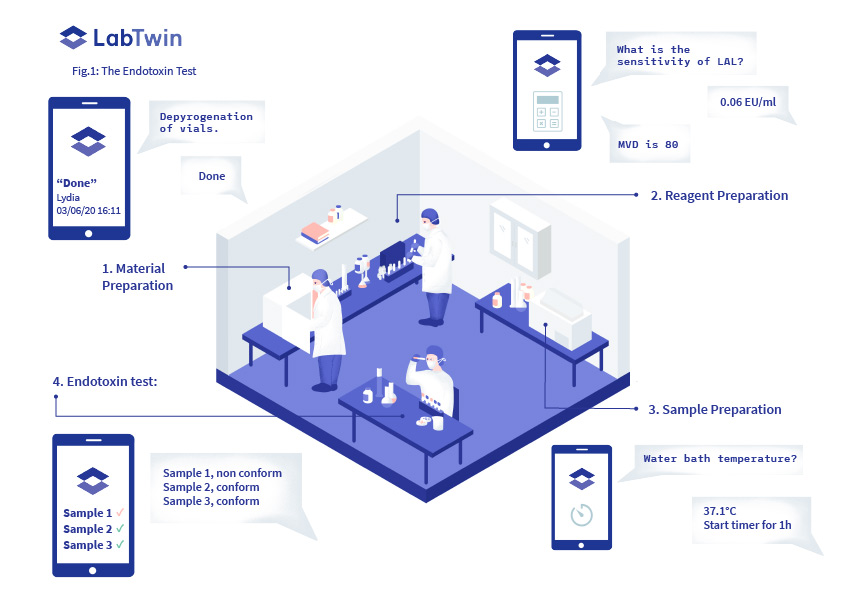
The Gel Clot Method is a simple test for the presence of endotoxins (Fig.1). Samples are aliquoted into test tubes and mixed with a reagent that causes endotoxin coagulation. If a gel clot forms in the sample tube, the sample is deemed contaminated, and the batch should be discarded.
Analysts must print standard forms for each assay and take the forms into the lab. They must report reagent lot numbers and expiration dates, all dilution calculations, incubation temperatures and times, and finally the result of each gel clot test.
In order to document this critical information, analysts need to interrupt their workflow every five minutes, remove their gloves and move from the bench to an isolated table where the form lies in order to prevent contamination. Upon test completion, each form must be dated and signed by both the analyst and the QA manager. It is then scanned and uploaded to the cloud.
With LabTwin’s voice-activated digital lab assistant, analysts can fill in digital QC forms from anywhere in the testing facility, simply by talking. Analysts can visualize each step of the form or LabTwin can read out results so they can be immediately verified. Furthermore, the digital lab assistant can provide automatic calculations, bypassing the necessary control of the QA manager for each calculation. LabTwin also validates that entered values are within an accepted range (e.g. 37°C ± 1°C) and provides integrated timers or timestamps to ensure precise time tracking.
“This is exactly how I thought LabTwin and LabTwin’s protocols would work.”
LabTwin User - Scientist Quality Operations, Top 10 Pharma Company
cGMP - SAFETY TESTING OF NON-STERILE SAMPLES: THE AGAR OVERLAY TEST
Non-sterile drugs, such as oral formulations, must satisfy microbiological purity criteria, ensuring that they do not exceed the maximum allowed microbiological count limits and do not contain prohibited microbes.
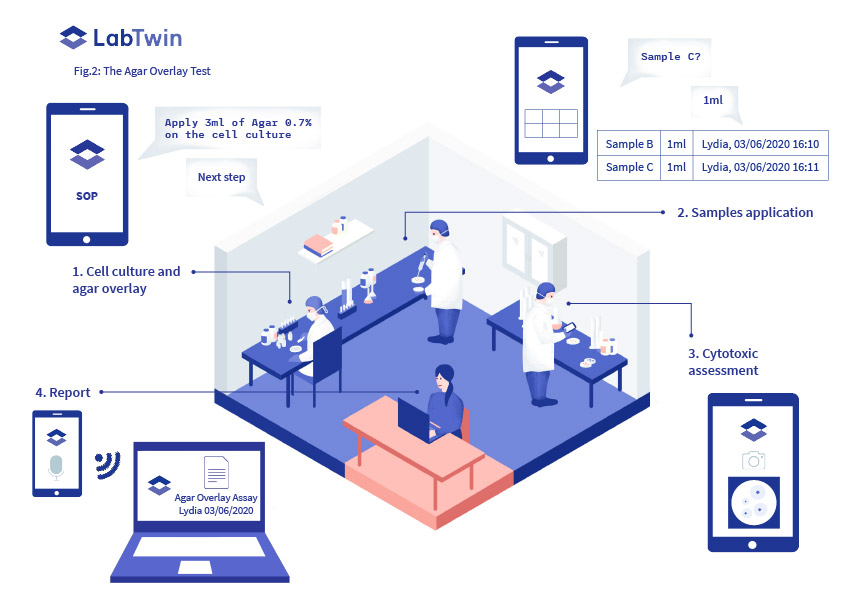
This can be assessed by performing an Agar Overlay or Agar Diffusion Test (Fig.2), where the potential cytotoxicity of the drugs is assessed.
Analysts must print standard forms and bring them into the lab so they can report lot numbers and expiration dates, as well as completion of each step of the protocol, with associated timestamps and assay results. Manual documentation is time-consuming and requires analysts to constantly interrupt their workflow.
LabTwin’s voice-activated digital lab assistant provides analysts with guided hands-free SOPs. The digital assistant also allows direct recording of all assay conditions and results, in real-time, by automatically translating voice notes, with timestamps and user identification, and by connecting directly with lab instruments. With LabTwin, analysts can also automatically generate comprehensive reports.
“My biggest challenge in the lab is constantly making decision to document right away or
to do it later and risk mistakes.”
LabTwin User - Lab Technician in Clinical Serology, Top 10 Pharma Company
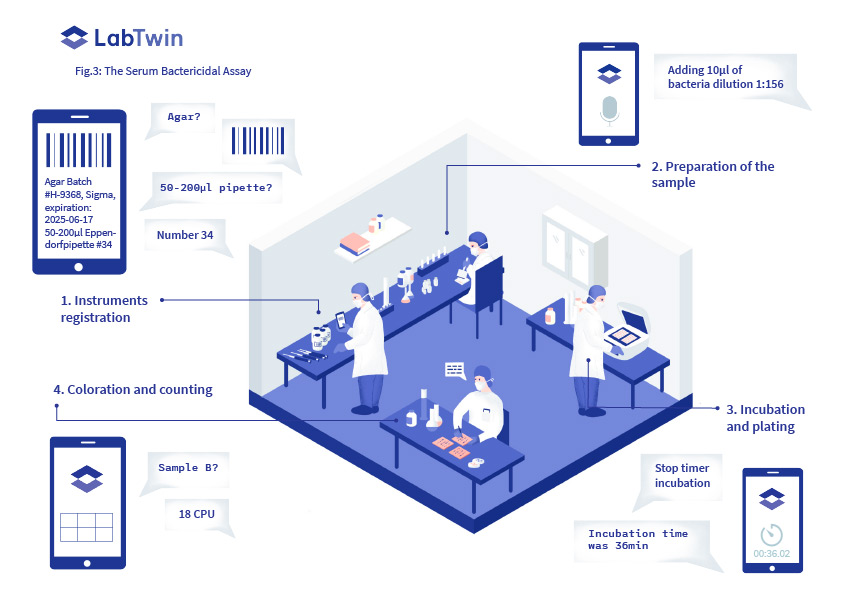
cGMP - EFFICIENCY TEST: SERUM BACTERICIDAL ASSAY (SBA)
Vaccine efficiency can be assessed by testing whether it can induce antibody production in test subjects, and by measuring the ability of newly created antibodies to bind and kill target microorganisms. Serum Bactericidal Assays are very valuable as they go beyond antibody quantification and test their biological function.
In the Serum Bactericidal Assay, the serum of tests animals or subjects is applied on the target bacteria cultures and the number of killed bacteria is quantified (Fig.3).
Analysts must document instrument identification numbers (including fridge, freezer, thermometer, pipette etc) as well as the lot number, expiration date and provider of each reagent. This takes a significant amount of time and must be completed before each assay is performed.
During testing, incubation times and temperatures, as well as all dilution calculations and assay results must be recorded. Each step is manually signed and checked.
To avoid contamination, forms are often kept in a separate part of the lab, and analysts have to go back and forth, degloving each time, to record this data. Otherwise, they must try to keep this information in their memory until the assay is completed.
The sensitive Serum Bactericidal Assay can present reproducibility issues as humidity or room temperature can play a significant role in the results.
Integration with MES software, LIMS, lab databases
With LabTwin guided input tables, analysts can record instrument and reagent information simply by scanning barcodes or by voicing their observations. Moreover, by integrating LabTwin with a LIMS, MES software or lab database, all reagents and samples details can be automatically linked to the current assay.
LabTwin’s voice-activated digital lab assistant can automatically record each protocol step with timestamps, allowing seamless workflow and preventing contamination. With the integration of a climate-sensor, LabTwin can also help with troubleshooting by systematically adding environmental information such as room temperature, pressure or humidity as metadata at each validated step.
“Half of my time is taken by documentation. And I run this assay three times a week!”
LabTwin User - QC Analyst, Top 10 Pharma Company
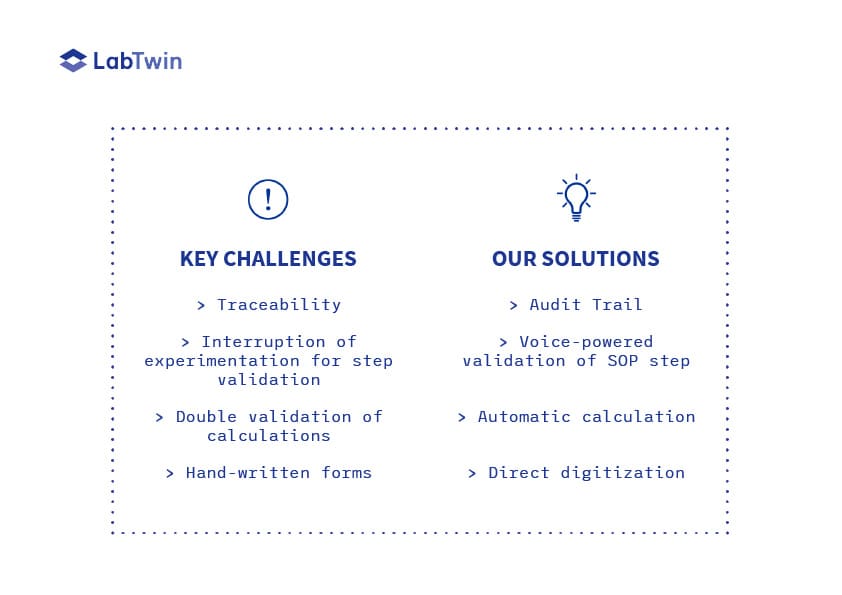
cGMP - COMMON CHALLENGES OF GMP TESTING
Microbiological testing in a GMP-regulated environment presents common and time-consuming documentation challenges:
- All reagents and instruments need to be recorded.
- Each experimental step should be confirmed by signatures and timestamps.
- All assay parameters and results have to be reported.
- Calculations have to be done manually, double checked by a second person and then reported 5. The forms and corresponding results need to be digitized and uploaded.
The widely used methods of manually completing paper forms and excel sheets at the point experimentation represents a heavy load for analysts who must constantly interrupt their workflow, deglove and write down the required information. This constitutes an important part of the assay duration, estimated by analysts to be between 20 and 50%.
Whenever calculations are involved, they must be verified by a second pair of eyes to reduce error rates. The presence of a second person to double check the work of an analyst represents a significant cost for the company.
Finally, digitization of a paper form by scanning prevents easy data access and fast computer-powered meta-analysis. This method creates data silos and is against FAIR principles.
“We have the goal to become a paperless lab and strongly believe that this is the future for our lab.”
LabTwin User - QC Project Leader, Top 10 Pharma Company
OUR SOLUTION
By allowing hands-free data capture at the point of experimentation, LabTwin’s voice-activated digital lab assistant saves a considerable amount of time and reduces the risk of contamination. All information is confirmed by voice input or barcode scanning, and is associated with electronic signatures and timestamps.
Calculations are directly supported and validated by LabTwin without the need for a second person. This saves time for both the analyst and the QA manager.
LabTwin automatically uploads all data into one central file which can be used to seamlessly create a report enriched with metadata that could have been lost or overlooked if manual recording methods are used. LabTwin’s digital assistant facilitates data access and sharing and prevents data loss and silos, thus contributing to FAIRification of the QC process.
LabTwin offers complete data security and data management compliance with data encryption, tiered access, private networks and complete audit trails.
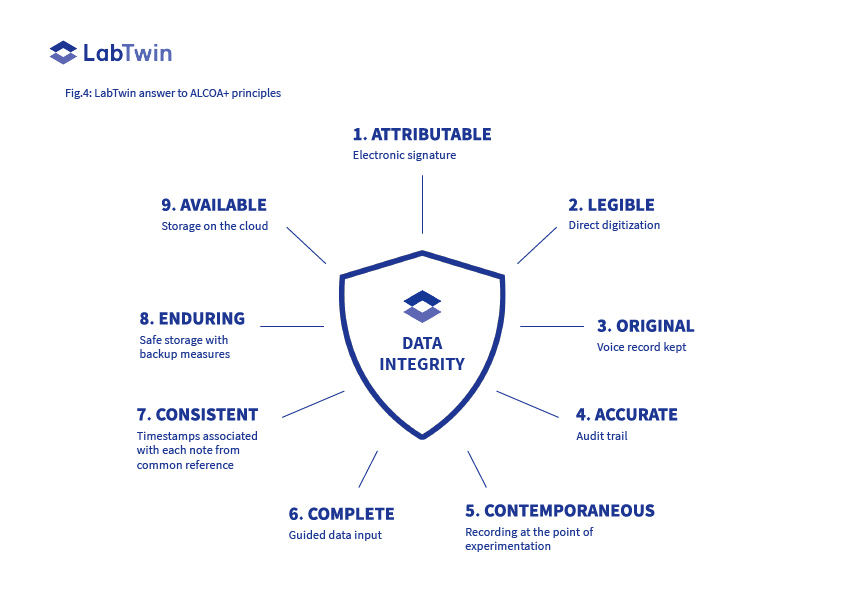
LabTwin is a ground-breaking solution enabling compliance with each of the ALCOA+ principles (Fig.4).