Data integrity is not a new problem and continues to be the major focus in regulated cGMP laboratories worldwide due to data falsification, poor data management practices, or ignorance of the regulations. Several regulatory agencies such as the Medicines and Healthcare products Regulatory Agency (MHRA), World Health Organization (WHO) Pharmaceutical Inspection Cooperation Scheme (PIC/S), and US Food and Drug Administration (FDA), as well as industry bodies such as the GAMP Forum, Parenteral Drug Association (PDA), and Active Pharmaceutical Ingredients Committee (APIC) have issued guidance documents on this subject (1-7). Accurate, legible, complete, and auditable data are essential for regulatory approval and manufacture of life science products and data integrity is an integral part of product development and quality control in the pharmaceutical and allied industries.
It is important for laboratories to assess their processes to identify and mitigate and/or remediate data integrity vulnerabilities. The WHO guidance document notes: 1.4: Mapping of data processes and application of modern Quality Risk Management (QRM) and sound scientific principles throughout the data life cycle (2).
The latest GAMP Good Practice Guide on Data Integrity by Design (8) recommends that data integrity be designed into a process using technical controls that can be applied consistently rather than a patchwork of procedural controls that are inconsistently applied. In assessment and remediation activities, many laboratories focus on computerized systems but often fail to consider data vulnerabilities inherent with paper-based processes that are manually documented such as sample preparation.
“Voice data input is a great technology for simultaneous working and data entry.”
Bob McDowall
Data Integrity Consultant and Director of R D McDowall Limited
PROBLEMS WITH PAPER RECORDS IN SAMPLE PREPARATION
Sample preparation consists of individual techniques such as weighing, dissolving, diluting, extracting, and incubating. Each analytical procedure has a sample preparation phase comprising one or more of these individual techniques linked together. The type of sample (e.g., solid, liquid) and the analytical technique (e.g., chromatography, spectroscopy) used to measure an analyte will determine which sample preparation techniques will be required. Owing to this variation it can be difficult to automate sample preparation for all analytical procedures. Therefore, most sample preparation is manual and recorded on paper consisting of mostly blank forms that are copied as needed by an analyst and uncontrolled. When preparing samples, technicians must stop work to record the various stages of the process, thus slowing workflows. Manual data collection also lacks built-in quality control and is prone to human error.
Additionally, when analytical instruments are used for sample preparation such as a balance for weighing samples and reference standard for analysis or preparation of buffers and mobile phases, it is used as a hybrid system with a paper record of the weights that are affixed to a paper record. According to the WHO guidance, hybrid systems are not to be encouraged and should be replaced (2).
Paper records have several data integrity issues associated with them, such as is the record original or is it the first passing result that has been submitted? Were the data recorded contemporaneously? Are the entries legible? In addition, recording on paper can be slow and the physical records are required for the second-person review.
As a result, data integrity guidance documents (3, 4) require stringent controls for blank forms necessitating each form to be uniquely numbered, printed so that they cannot be copied, and issued and reconciled, thus creating a high administrative burden for laboratories that want to continue to use paper.
SAMPLE PREPARATION FOR CHROMATOGRAPHIC ANALYSIS
This is shown in Figure 1 where a uniquely numbered and controlled analytical worksheet is used to record how a sample is prepared for chromatographic analysis.
> Samples and reference standards are weighed on an analytical balance and the printout is affixed to the worksheet by the analyst and signed and initialed over the edge to prevent replacement later by a different printout.
> Each weighed sample or reference standard is transferred to a volumetric flask, dissolved, and made up to volume.
> Each flask is diluted by taking an aliquot from each flask, transferring it to another labeled volumetric flask, and making up to volume.
> A portion is transferred to a labeled high performance liquid chromatography (HPLC) vial ready for transfer into the chromatograph autosampler for analysis.
Recording these activities on the controlled worksheet by hand is slow and sometimes doubles the preparation time.
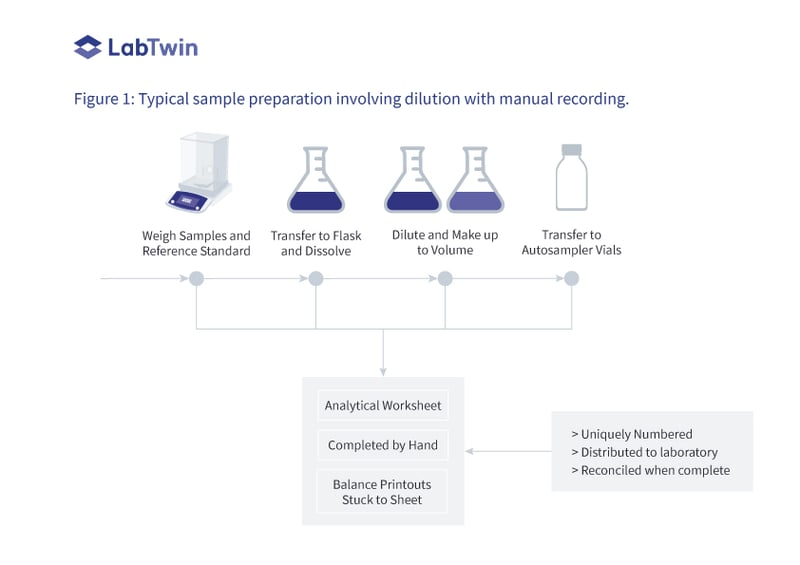
Figure 1: Typical sample preparation involving dilution with manual recording.
PREPARATION OF A BUFFER SOLUTION
A similar situation can be found when preparing a buffer for use in either sample preparation or as a component of HPLC mobile phase. This process is shown in Figure 2 where an analytical balance and a pH meter are the instruments involved in the process and a laboratory notebook is used to record the work performed.
> The process starts with weighing the materials that comprise the buffer on an appropriate balance.
> The chemicals are transferred to a volumetric flask and dissolved according to the instructions for preparing the buffer.
> Sufficient water is added and the buffer pH is measured.
> If appropriate, the buffer pH is then adjusted within the required range.
> The volume of the flask is made up to volume and the buffer is transferred to a labeled storage container.
All the work is recorded in a bound and paginated laboratory notebook. Like the previous example, the instrument printouts are stuck onto notebook pages and signed or initialed as before. Again, it is a slow and manual process. Moreover, all physical records must be reviewed by a second person.

Figure 2: Preparation of a buffer solution.
IS THERE A BETTER WAY TO RECORD LABORATORY WORK?
There needs to be a better way to ensure data integrity that is flexible enough to accommodate different types of operations, interface with analytical instruments, and automate the documentation process. One way to achieve this is to use voice data entry via a Digital Lab Assistant.
“By connecting directly with lab instruments and databases, and allowing lab staff to record data by speaking, Digital Lab Assistants make documentation a natural, automated, and digitized process. Full capture of data and metadata makes it much easier to reproduce experiments and troubleshoot any errors or deviations,” says Steffen Gloth, Co-founder and Head of Operations of LabTwin.
LabTwin’s Digital Lab Assistant eliminates the need for paper records by allowing analysts to record data using their voice. The software can also interface directly with analytical instruments and transfer instrument readings to the database, ensuring data integrity. The Digital Lab Assistant can also identify an individual analyst by their voice, and automatically identify who enters which data into the system database. This provides several advantages from both business efficiency and data integrity perspectives. Information can be recorded while the analyst is performing their work without the need to stop and write down data. Therefore, all entries are contemporaneous with accurate user identification as well as date and time stamps.
“Voice data input is a great technology for simultaneous working and data entry,” says Bob McDowall, a data integrity consultant and Director of R D McDowall Limited.
“By connecting directly with lab instruments and databases, and allowing lab staff to record data by speaking, Digital Lab Assistants make documentation a natural, automated, and digitized process. Full capture of data and metadata makes it much easier to reproduce experiments and troubleshoot any errors or deviations.”
Steffen Gloth
Co-founder and Head of Operations, LabTwin
USING VOICE ENTRY TO AUTOMATE SAMPLE PREPARATION
Figure 3 shows the same laboratory process to prepare samples for HPLC analysis as shown in Figure 1 but with a Digital Lab Assistant to record the work as an analyst proceeds through their tasks.
> Each sample weight is captured directly from the analytical balance and transferred into the database. Sample information is dictated into the database at the same time.
> The work of dissolving and diluting each sample along with the transfer to the HPLC vial can also be recorded by the analyst as they work.

Figure 3: Sample preparation with recording of work by a Digital Lab Assistant.
VOICE DATA ENTRY FOR BUFFER PREPARATION
Voice automation of the process to prepare a buffer is shown in Figure 4 where both the balance and the pH meter used are interfaced with the system.
> Weights of the chemicals are captured by the balance and transferred to the database along with voice input of the identities, lot numbers, and expiry dates.
> The checks and pH adjustment of the solution are captured directly from the instrument together with the verbal description of work performed by the analyst.
Compare this with the original process shown in Figure 2 to see the improvement in recording activities, which ensures the integrity of generated data.

Figure 4: Preparation of a buffer solution with recording of work by a Digital Lab Assistant.
ADVANTAGES OF USING VOICE INPUT FOR cGMP RECORDS
There are several advantages of using voice input to record cGMP activities:
> Voice capture can be performed at the same time as sample preparation work, thus generating original and contemporaneous records with automated time and date stamps linked to a trusted time source.
> Attribution of action is improved and continually verified with the voice recognition software that is trained for accurate voice-to-text conversion.
> Original records are created as the work is performed and stored on enduring and secure media.
> Access to records stored in the database for second-person review, inspections, and audits is much easier than finding paper records.
> Data capture from analytical balances and other analytical instruments eliminates paper printouts and meets the requirements of the WHO and PIC/S guidance documents (2, 3).
> Data captured can include the whole balance weighing sequence with the appropriate metadata.
> There are also improvements in productivity as capturing data hands-free avoids many distractions and improves the overall productivity of sample preparation, which is one of the most manual processes in any laboratory.
“LabTwin's voice-activated digital lab assistant ensures data integrity with automatic user attribution, time and date stamps, allowing an audit trail at every stage of research, development and production.”
Denis Özdemir, PhD
Head of Customer Services, LabTwin
REFERENCES
-
MHRA GXP Data Integrity Guidance and Definitions. 2018, Medicines and Healthcare products Regulatory Agency: London.
-
WHO Technical Report Series No.996 Annex 5 Guidance on Good Data and Records Management Practices. 2016, World Health Organization: Geneva.
-
PIC/S PI-041-3 Good Practices for Data Management and Integrity in Regulated GMP / GDP Environments Draft. 2018, Pharmaceutical Inspection Convention / Pharmaceutical Inspection Cooperation Scheme: Geneva.
-
FDA Guidance for Industry Data Integrity and Compliance With Drug CGMP Questions and Answers. 2018, Food and Drug Administration: Silver Spring, MD.
-
GAMP Guide Records and Data integrity. 2017, International Society for Pharmaceutical Engineering: Tampa, FL.
-
Technical Report 80: Data Integrity Management System for Pharmaceutical Laboratories. 2018, Parenteral Drug Association (PDA): Bethesda, MD.
-
Practical risk-based guide for managing data integrity, version 1. 2019; Available from: https:// apic.cefic.org/pub/Data_Integrity_Best_Practices_Guide_for_API_FINAL_March-2019.pdf.
-
GAMP Good Practice Guide: Data Integrity by Design. 2020, International Society for Pharmaceutical Engineering: Tampa, FL.



