The pharmaceutical and biotech industries are highly regulated to ensure drugs and other therapeutic products are safe and effective. Pharmaceutical companies must follow current Good Manufacturing Practice (cGMP) regulations throughout all stages of drug manufacturing.
Data integrity is an essential part of GMP regulations. In fact, regulatory inspectors from the U.S. Food and Drug Administration (FDA), the U.K Medicines and Healthcare products Regulatory Agency (MHRA), and other regulatory agencies focus on data integrity during audits and inspections.
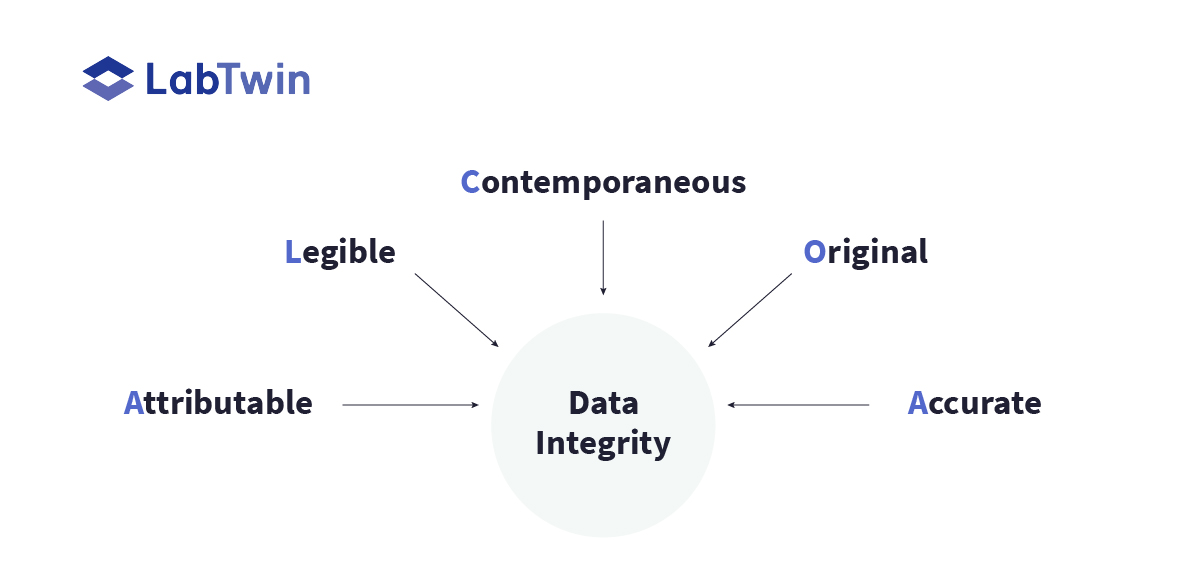
The FDA data integrity guidance refers to the ALCOA+ principles of data management.
To maintain data integrity, companies need to make sure that data are:
- Attributable – easy to see who created the data record
- Legible – easy to read
- Contemporaneous – created while an operation/procedure takes place
- Original – a true, unmodified record
- Accurate – reflects what really happened during the operation/procedure
Since the ALCOA principles were first developed, several new requirements have been added. These are captured in the ‘+’ part of ALCOA+, and include the need for data to be:
- Available – the right people can access the data
- Enduring – the data are stored securely and cannot be deleted
- Complete – the data cover all aspects of manufacturing
- Consistent – all data are managed in the same way
If pharmaceutical companies can follow ALCOA+ principles of data management, it is much easier to prove data integrity during regulatory audits. However, it is not simple to implement a data management system robust enough to adhere to the ALCOA+ principles.
In GMP environments, it can be particularly difficult to collect contemporaneous data and metadata. Technicians cannot manually record sample preparation conditions, for example, while their hands and eyes are busy. In labs using paper records, an electronic lab notebook (ELN) or a manufacturing execution system (MES), technicians must either stop their sample prep to complete the required data records or try to remember all conditions and update records after they have finished preparing their samples. Both these choices increase the chance of human error.
Furthermore, cGMP environments may require a second person to double check calculations and documentation of key manufacturing processes. This obviously leads to increased staffing and slows down the manufacturing timeline.
Voice-activated digital lab assistants allow technicians to collect data directly from lab instruments and make voice notes while they work. Therefore, digital lab assistants make it easy to create accurate, legible, contemporaneous data in cGMP environments. With voice-powered assistants, data is also attributable via user identification, electronic signatures and automatic time and date stamps.
To learn more about how voice entry can improve ALCOA+ GMP data integrity, read this white paper.











